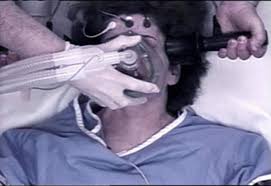
The Food and Drug Administration (FDA) has initiated a proposal that would reclassify Electroconvulsive Therapy Devices (ECT) from its highest risk category III to allow electric shock machines to be utilized in the treatment of specific alleged mental illnesses with less regulatory controls.[1] This is despite the federal agency’s admission that the ECT device has not been proven safe and effective. To date, nearly five million Americans have received ECT “treatment” without ECT manufacturers being required to submit valid scientific evidence, such as clinical trials, of the device’s safety or effectiveness.[2]
The proposal has reignited a firestorm that the FDA has colluded with the American Psychiatric Association (APA) to promote a dangerous treatment and protect the fiscal concerns of APA members rather than protect patient lives.
It is no secret that the FDA has procrastinated for more than five years over this proposal after first entertaining the idea of reducing the device’s risk category in 2009, when it requested public input on this. At a public hearing in January 2011, it was heard that nearly 80 percent of the respondents and a further 92 group submissions representing more than 6000 individuals, were against reclassification.[3]