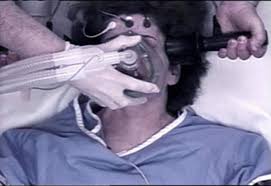
The Food and Drug Administration (FDA) has initiated a proposal that would reclassify Electroconvulsive Therapy Devices (ECT) from its highest risk category III to allow electric shock machines to be utilized in the treatment of specific alleged mental illnesses with less regulatory controls.[1] This is despite the federal agency’s admission that the ECT device has not been proven safe and …
Tag Archive
Below you'll find a list of all posts that have been tagged as “PTC Therapeutics”